Introduction
Post-transplant cyclophosphamide (PTCy)-based prophylaxis for acute graft versus host disease (aGvHD) has become a standard protocol in HLA haploidentical bone marrow transplantation. Preliminary evidence indicates that compared to the standard dosage (50 mg/kg on days +3 and +4 post-transplantation), a regimen of half the typical cyclophosphamide dosage is both feasible and effective. However, the optimal dosage and timing of PTCy for aGvHD prophylaxis in a HLA identical setting, using peripheral stem cells as the graft, remain unknown. This study aims to investigate the efficacy and safety of a reduced PTCy dosage regimen in patients undergoing peripheral blood stem cell transplants (PBSCT) with HLA indentical donors.
Methods
This prospective, single-center phase I/II study enrolled patients scheduled for PBSCT with HLA identical donors. The aGVHD prophylaxis regimen comprised PTCy on days +3 and +4, followed by cyclosporine initiation from day 5 post-transplant. In the phase I stage, an initial cohort of five patients received PTCy at a dosage of 50 mg/kg/day on days +3 and +4 (designated as dose level 1, DL1). This was followed by a 3+3 dose de-escalation design with subsequent dose levels as follows: DL2, 50 mg/kg on day +3 and 25 mg/kg on day +4; and DL3, 25 mg/kg on both day +3 and +4. The dose-limiting toxicity for this de-escalation was defined as the occurrence of grade III-IV aGVHD within the first 100 days post-transplantation.
The phase II stage comprised an expansion cohort, which was based on the optimal dose identified during phase I. aGvHD classification was established using the Mount Sinai Acute GVHD International Consortium (MAGIC) criteria, while chronic GvHD (cGvHD) was assessed per the latest National Institutes of Health Consensus on cGvHD. Patients were closely followed-up at +1, +2, +3, +4, +6, +9, +12, +18, and +24 months post-transplant.
Results
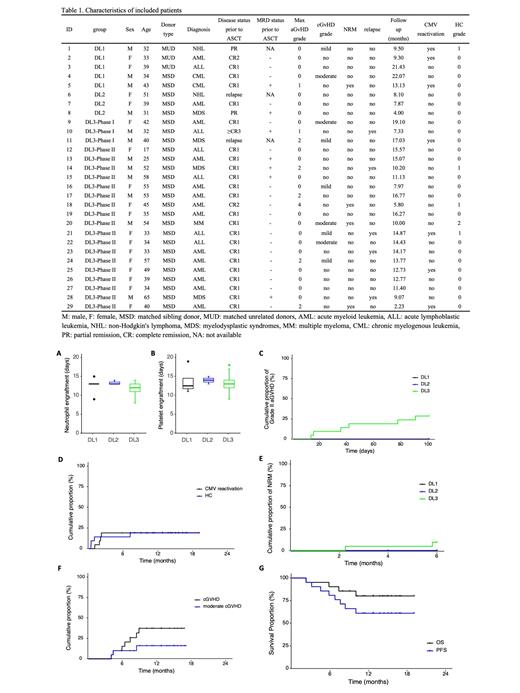
In the first stage, a total of 11 patients (5 in DL1, 3 in DL2, and 3 in DL3) were included, and no patient developed grade III-IV aGVHD. Based on this, DL3 was selected as the PTCy dose for phase II, which comprised 18 patients. Overall, 29 patients (17 females, 12 males) with a median age of 39 years (range 17-65) were included, and the median follow-up duration was 12.7 months (range 2.2-22.1). Patient and disease characteristics are summarized in Table 1. By day +30 post-ASCT, all patients achieved complete remission (CR) and minimal residual disease (MRD) clearance. No patient developed grade III-IV aGVHD within 100 days post-transplantation.
There was no significant difference in the neutrophil and platelet engraftment interval among the three groups (Figure 1A, B). Among the 21 patients who received the DL3 dose of PTCy, the estimated cumulative incidence of grade II to IV aGVHD at day +100 was recorded at 28.6%, and the incidence of aGVHD at day +100 presented no difference among the three DL groups (Figure 1C). In addition, 1 patient in DL3 developed grade IV intestinal aGVHD on day +141 and died one month later. The estimated 1-year cumulative incidence of hemorrhagic cystitis (HC) and cytomegalovirus (CMV) reactivation was 19.3% and 19%, respectively (Figure 1D). Five patients (all in DL3) experienced relapse, with a median interval of 6.7 months and an estimated 1-year cumulative incidence of 10.6%. A total of 5 fatalities occurred during our study (1 in DL1, and 4 in DL3), 1 due to relapse, 2 from COVID-19-associated pulmonary aspergillosis, 1 from severe septicemia and 1 due to grade IV aGVHD as mentioned above. The estimated 6-month cumulative incidence of NRM was 9.5%, presenting no difference among the three DL groups (Figure 1E). Among the 20 evaluable patients, the estimated 1-year cumulative incidence of chronic GVHD (cGVHD) and moderate cGVHD was 37.3% and 16% (Figure 1F), respectively, with no severe cGVHD observed. No cases of patient relapse were reported. The estimated 1-year progression-free survival (PFS) and overall survival (OS) were 61.2% and 80.4%, respectively (Figure 1G).
Conclusion
The findings of this study substantiate that a two-day regimen of PTCy at 25 mg/kg/day serves as an effective and safe prophylactic measure against aGVHD, comparable to the standard dosing protocol. However, to definitively establish whether the de-escalated PTCy dosage provides superior outcomes to the standard regimen, longer-term follow-up and comparative studies are warranted.
Disclosures
No relevant conflicts of interest to declare.